
Clinical use in tissue repair and organ transplantation will become a reality
3D bio-printing is believed to bring about a huge revolution to the modern medicine.
Imagine in the future, patients in desperate need of organ transplantation no longer have to go through an endless waiting for the matching organs, nor do they have to tolerate the excruciating pain caused by transplant rejection. Physicians only need to use the cells of the patients as raw materials in order to cultivate tissues and organs that are completely suitable for patients. This technology, once implemented in large scale, will bring a new life to numerous patients needing organ transplatation and tissue repair. With the development of 3D bio-printing technology, this future will become a reality soon.
In fact, the “tissue repair robotics” project previously announced by RunYoung is closely related to 3D bio-printing technology
I. What is 3D Bio-printing?
As a branch of regenerative medicine, 3D bio-printing has transformed from a futuristic technology that “seems very cool” to a tangible technology that we can see with our own eyes.
This technology uses the three-dimensional model as the “blueprint” and 3D printer and bio materials to produce customized grafts, auxilliary grafts, scaffolds, tissues and organs, and other products from the biological ink containing cells and biological materials, so as to realize the tissue functionalization.

Similar to the mechanism of 3D printing, 3D bio- printing builds a three-dimensional object by stacking layers of materials one at a time. Unlike ordinary 3D printers, which use metal, plastic or ceramic materials, 3D bioprinter uses “bio-ink” “: A printable material containing living cells. Many biological inks comprise of water-ruch molecule called “hydrogel”. The hydrogel is mixed with millions of living cells and various chemicals and is used to promote cell communication and growth. Some biological inks only contain single cells, and some combine many different cells to create more complex biological functional structures.
Generally speaking, 3D bioprinting means to print biocompatible materials containing living cells into three-dimensional functional living tissues through 3D printing technology, and realize functions similar to, identical with, or even superior to target tissues or biological organs.
It is fair to say that 3D biological printing is one of the most promising emerging technologies for manufacturing human organs in vitro.
II. Prospect of 3D Bioprinting Technology
Since the development of the first bioprinter at the beginning of the 21st century, bioprinting has entered many fields including pathological model production, tissue design for drug screening, medical research and regenerative medicine.
When 3D bioprinting technology was first applied in biomedicine, only 3D scaffolds including bone, cartilage, blood vessels, nerves, tendons, teeth and skin tissues could be printed. Nowadays, its application has gradually expanded to producing organ prototypes with more complex structures and biological components, such as liver, kidney and heart. Up to now, 3D biological printing technology has gradually matured in scientific research and application fields, including:
- Biomimetic tissues and organs: such as spinal cord, heart, skin printing, etc;
- Tissue engineering scaffold: such as bone repair scaffold, meniscus scaffold, bone cartilage scaffold, etc;
- Cell research and treatment: such as cell immune research, embryonic stem cell printing, neural cell printing, etc;
- Personalized drug testing model: such as organ chip, personalized liver cancer model, brain like model for neurodrug screening;
- High end medical devices: such as anti HPV protein sustained release stent, heart stent, vascular stent, artificial heart valve, etc;
- Development and application of advanced materials: such as artificial meat.
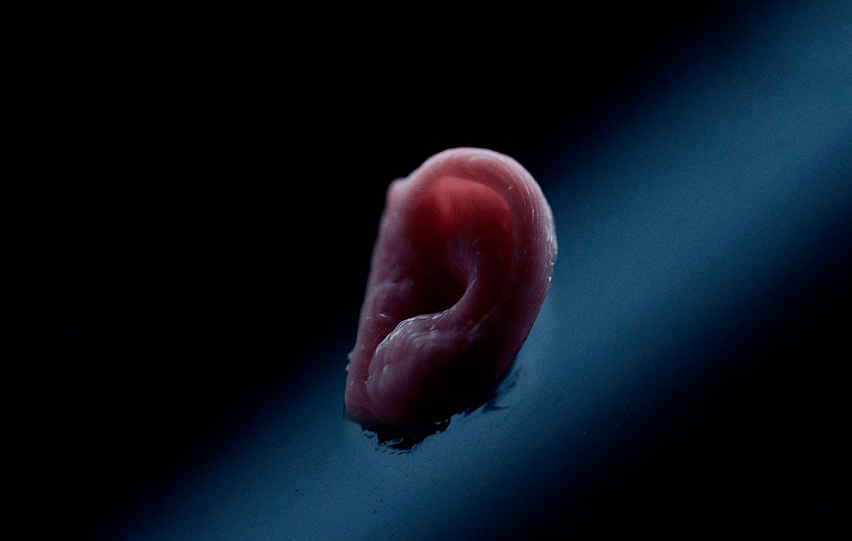
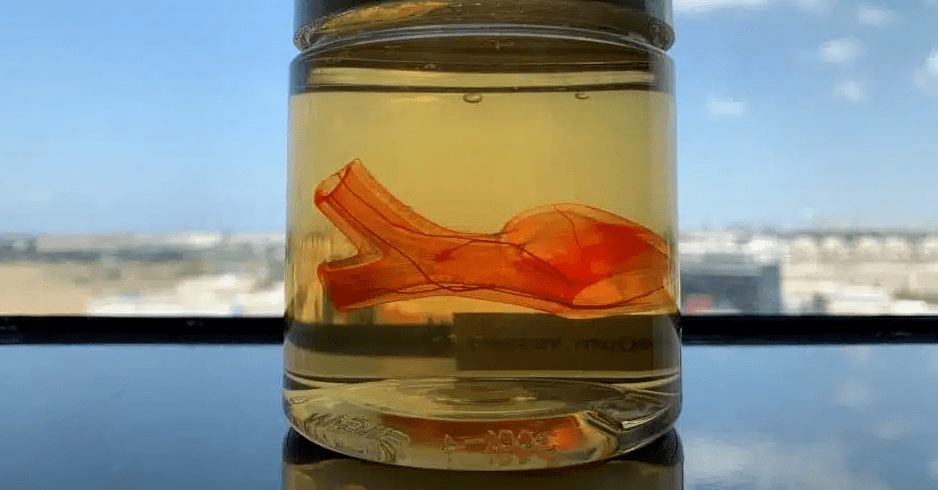
If we compare human body to a intricate machine, the ultimate role of 3D bioprinting is similar to the technologies that people use to manufacture “spare parts” that can replace the “faulty parts” of this “machine” (organ transplantation). However, at the current stage, it can only be used to make some simulation models (in vitro models) used to simulate the process of “locating fault parts”. To be more exact, we still have a long way to go before realizing the its ultimate purpose of “faulty parts replacement” with the clinical replacement of patients’ functional organs as its end point.
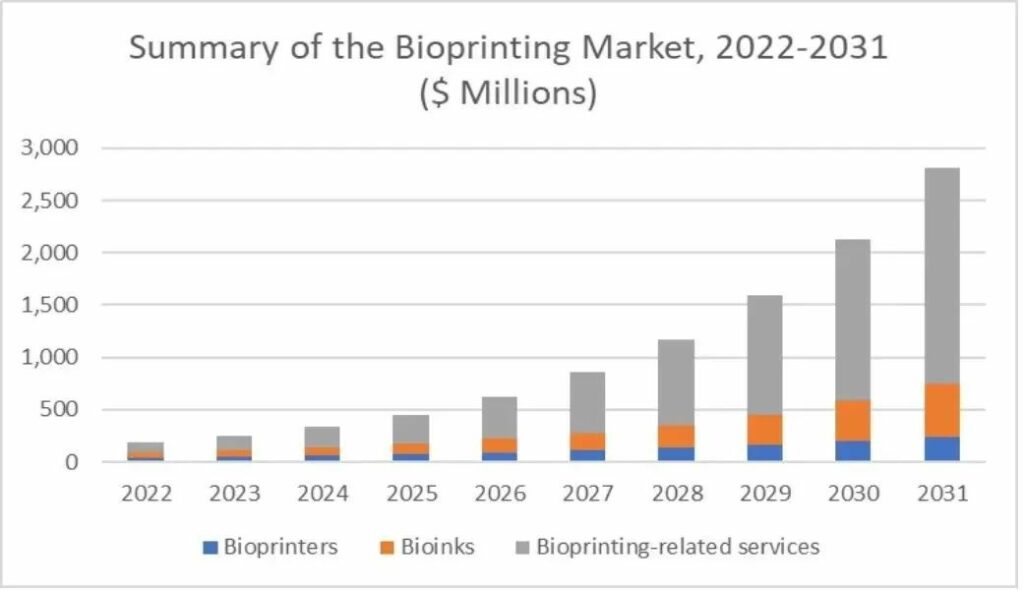
Despite still in its initial stage, this technology is expected to bring about disrumptive changes to the healthcare and pharmaceutical industries: all major market analysis and prediction institutions around the world have seen the considerable market potential of 3D bioprinting technology, and have made their own 5- to 10-year market estimation of this technology.
| Forecaster | Total Addressable Market (USD) | Year of Completion |
| BIS | 4.7 billion | 2025 |
| Grand View Research | 4.1 billion | 2026 |
| Market Research Future | 1.923 billion | 2023 |
| Big Market Research | 1.876 billion | 2025 |
| IDTechEX | 1.9 billion | 2023 |
| SmarTech | 2.7 billion | 2031 |
Conservative estimation shows that, within next 10 years, this technology will have a global TAM of more than US $2 billion. North America currently has the largest market share by region and its advantage will remain for the following 5-10 years. However, the Asia-Pacific region will have a higher growth rate in the next 5 years, and will be one of the main markets for biological 3D printing in the future.
III. The Key and Major Obstacles to the Development of 3D Bioprinting Technology
At present, the research of 3D bioprinting technology in the field of tissue engineering is still in its infancy. How to use 3D printing technology to construct living tissue structure is still a difficult task. Therefore, successful 3D bioprinting must overcome the following specific technical problems.
1. Cell Technology
What kind of cells to choose is crucial for the biological tissues or organs produced by 3D bioprinting. Different tissues of the human body are composed of unique cells. The liver mainly contains liver cells, the heart mainly contains myocardial cells, and the skin mainly contains various epithelial cells. However, not all tissue-specific cells can be isolated and cultured in vitro, and not all cells can maintain their biological activity after being exposed to the 3D bioprinting environment. Therefore, how to optimize cell idolation, culture and proliferation technology is the precondition to ensure the success of 3D biological printing.
2. Biomaterials (aka. Bio ink)
The “biomaterial (bio ink)” required for printing is one of the most important factors that affect and restrict the development of 3D biological printing. Different tissues and organs of the human body have their own physical and mechanical characteristics. The softness of skin tissue and the hardness of bone tissue lead to the need to select biomaterials corresponding to tissue characteristics during 3D printing of different tissues, and these materials need to maintain the biological activity and function of selected cells to the greatest extent. More importantly, the selected materials must be able to be operated through a 3D printing system. This makes the selection of biomaterials very challenging, and requires continuous optimization and improvement in the printing process. On the other hand, high-quality and suitable materials are often difficult to obtain and extremely expensive, which will be another major problem to overcome in the process of technological development. Therefore, biological 3D printing still has many technical problems that need to be addressed, and requires multidisciplinary and interdisciplinary cooperation.
3. Manufacture Platforms
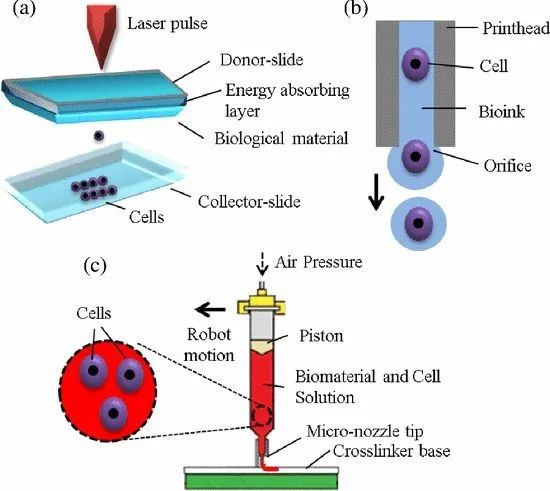
Currently, the main platforms used for 3D bioprinting falls in the following three categories: Laser-based, Inkjet-based, and Extrusion-based. The three platforms have their own advantages and disadvantages, different special functions and requirements for hardware technology. How to make different tissues and organs accordingly and how to reasonably choose the platform are also the key to the success of 3D bioprinting. These three platforms have been used in traditional printing or 3D manufacturing systems, and the critical point is how to integrate them into the biological manufacturing system, so that they can play the maximum role, while maintaining the biological activity of cells and the physical properties of biological materials, and making products meet the standards of medical applications, which must be overcome and constantly optimized.
4. Vein System
Another important point is that most human tissues and organs have their own vascular systems and need to get enough blood supply to maintain bioactivities. However, the current 3D bioprinting technology is not yet able to produce substitutes that have the same functions as the human vascular system, let alone integrating the vascular system into 3D printed tissues. Without the vascular system, the 3D printed organs will not be able to survive for a long time, and our aspiration of using 3D printed organs to rescue patients with organ failure will be just a fantasy. Therefore, 3D bioprinting of tissues and organs with vascular system that enables them to integrate into the whole blood circulation system of the human body is the biggest challenge and the ultimate goal of this industry.
IV. “In Situ Bioprinting” As The More Advanced Clinical Application of 3D Biopriting
Nowadays, the grafts are printed in vitro before transplantation. However, a more advanced application of 3D bioprinting technology that will revolutionaize the current clinical practice is to realize tissue repair and organ replacement in vivo. To achieve this, we need to be aware of shortcomings of the existing bioprinting technology:
- It is difficult for the current technology to achieve the irregular shape required for actual clinical tissue repair, and the printed matter is easy to degrade and deform after in vitro cultivation;
- The existing bioprinting hydrogel materials are difficult to sew and fit, and need chemical modification to be well bonded, and chemical modification and adhesive will also significantly reduce the regeneration effect;
- The printing process is complex and slow (usually several hours), which not only leads to slow response to traumatic time and reduces the regeneration effect, but also patients often need a second operation;
- The printing process is complex and slow (usually several hours), which not only leads to slow response to traumatic time and reduces the regeneration effect, but also patients often need a second operation;
To conquer the abovementioned challenges, researchers have developped a biological printing method that has recently attracted wide attention: directly printing bio ink into the patient’s body, namely “in situ biological printing”, also known as “in vivo bioprinting”.
Compared with in vitro bioprinting, in situ bioprinting has the following advantages:
- Doctors can conduct treatment and control the process in real time, and ensure that the printed matter can be accurately matched with the injury;
- In situ crosslinking can effectively improve the combination degree of prints and residual tissues;
- Compared with in vitro culture, human body can provide better conditions for cell regeneration and infection reduction;
- In addition, the flexibility of in situ printing is very high. Even if there is no professional 3D bioprinter, in some emergencies (battlefield, traffic accidents, etc.), manual operation or direct injection of biological ink can also be used as an alternative.
The “tissue repair robot” jointly built by RunYoung and the godfather of surgical robotics is exactly aiming at in situ bioprinting technology. Through the development of minimally invasive robotic surgery system, 3D tissue printing will be realized within the human body.
Through the cooperation with the godfather of surgical robotics and with the leading innovation experts in biomaterial research, the product has applied for patent in USA and its prototype has been successfully manufactured. It is expected that in situ bioprinting in small animal models will happen in 2022-23, and in situ bioprinting in large animal models will take place in 2024-25.
With the increasingly vigorous development of 3D bioprinting technology, more biomedical applications are expected to arise in the next few years. RunYoung will be committed to working with industrial partners, universities and research institutes to create a path for disruptive and innovative breakthroughs in the field of 3D bioprinting, and turn 3D bioprinting into a tangible future by pushing its wide application in clinical practice!